Hydrogen
It is the lightest and most abundant element in the universe, constituting about 75% of its elemental mass. In its most common form, hydrogen exists as a diatomic molecule (H2), which is a colorless, odorless, and tasteless gas.
Hydrogen is highly reactive and can form compounds with almost all other elements.
It is used in a wide range of industrial applications, such as in the production of ammonia for fertilizers, in petroleum refining, and in the manufacture of various chemicals. It is also used as a fuel for vehicles, either in its pure form or as a component of other fuels like natural gas.
Hydrogen has attracted attention in recent years as a potential clean energy source. When hydrogen is burned or reacted with oxygen in a fuel cell, it produces only water and releases a significant amount of energy.However, the production of hydrogen itself often involves the use of fossil fuels, so it is not yet a completely emissions-free energy source. Efforts are underway to develop methods of producing hydrogen from renewable sources like solar and wind power.
Who Introduced Hydrogen?Hydrogen has been known since ancient times, but it was not recognized as a distinct element until the late 18th century.
In 1766, the English chemist Henry Cavendish discovered that a gas produced by the action of sulfuric acid on metals was distinct from all other gases known at the time, and he named it "inflammable air." Cavendish also determined several of its properties, including its low density and flammability.
In 1783, the French chemist Antoine Lavoisier proposed the name "hydrogen" for the gas, which is derived from the Greek words "hydro" (meaning "water") and "genes" (meaning "forming").
Lavoisier recognized that hydrogen was an essential component of water, and he showed that when hydrogen was burned, it combined with oxygen to form water.
This was an important step in the development of the modern concept of chemical elements, which holds that all matter is made up of atoms of different elements that combine to form compounds Chemical Reactions and Equation Of Hydrogen:-
The chemical equation for hydrogen depends on what reaction or process you are considering. Here are some common chemical equations that involve hydrogen:
The combination of two hydrogen atoms to form a hydrogen molecule (H2):
H + H → H2
The reaction of hydrogen gas with oxygen gas to form water:
2H2 + O2 → 2H2O
The reaction of hydrochloric acid (HCl) with zinc (Zn) to produce hydrogen gas and zinc chloride (ZnCl2):
Zn + 2HCl → ZnCl2 + H2
The reaction of sodium hydroxide (NaOH) with aluminum (Al) to produce hydrogen gas and sodium aluminate (NaAlO2):
2Al + 2NaOH + 6H2O → 2NaAlO2 + 3H2
These equations are examples of some of the many reactions and processes that involve hydrogen. The chemical equation for a particular reaction or process will depend on the specific reactants and conditions involved.
Why We Need Hydrogen Gas?Hydrogen gas has a number of important applications and uses in various industries and fields.
Here are some reasons why we need hydrogen gas:
Energy production: Hydrogen is a potential source of clean energy, as it can be burned to produce heat and electricity with water as the only byproduct.
Fuel cells, which use hydrogen and oxygen to produce electricity, are being developed as an alternative to conventional batteries for use in electric vehicles and other applications.Industrial processes: Hydrogen is used in a wide range of industrial processes, such as the production of ammonia for fertilizers, in the refining of petroleum, and in the manufacture of various chemicals and materials.
Food production: Hydrogen is used in the production of vegetable oils and margarine, as well as in the hydrogenation of sugars and other food products.
Metal processing: Hydrogen is used in the production of metal alloys, such as in the production of titanium and other metals, as well as in the welding of metals.
Electronics: Hydrogen is used in the production of electronic components, such as in the manufacturing of semiconductors and flat panel displays.
Overall, hydrogen gas is a versatile and important substance that has a wide range of applications and uses in industry, energy production, and various other fields. Efforts are underway to develop methods of producing hydrogen from renewable sources to reduce emissions and environmental impact.
Unknown Facts About Hydrogen:Here's an unknown fact about hydrogen:
Despite being the most abundant element in the universe, hydrogen is actually quite rare on Earth in its pure form. This is because hydrogen is a very light gas and tends to escape the Earth's atmosphere, and it also tends to react with other elements to form compounds.
However, even though it is rare in its pure form, hydrogen is a key component of many substances that are abundant on Earth, such as water (H2O) and hydrocarbons (compounds that contain both hydrogen and carbon, such as methane and gasoline).
Another interesting fact about hydrogen is that it was the first element to be recognized as such. In 1766, the English chemist Henry Cavendish discovered a gas that he called "inflammable air," which was later identified as hydrogen.
This discovery helped pave the way for the development of modern chemistry and our understanding of the nature of matter. Why Hydrogen is Placed in Group 1 in Periodic TableHydrogen is placed in group 1 of the periodic table due to its electronic configuration and chemical properties.
Group 1 of the periodic table, also known as the alkali metals, includes lithium (Li), sodium (Na), potassium (K), rubidium (Rb), cesium (Cs), and francium (Fr).
These elements all have one valence electron in their outermost energy level, making them highly reactive and giving them similar chemical properties.
Hydrogen, although it is not a metal, has one valence electron in its outermost energy level and exhibits similar chemical properties to the alkali metals. For example, it can lose its single electron to form the H+ ion, which is similar to the Na+ ion in sodium. However, hydrogen also exhibits unique chemical properties that differentiate it from the alkali metals, such as its ability to form covalent bonds with other nonmetals. Because of this, some periodic tables place hydrogen in its own group, separate from the alkali metals. The placement of hydrogen in the periodic table has been a topic of debate for many years, and there is no one universally accepted placement. Some periodic tables place hydrogen in group 1, while others place it in group 17 (the halogens) or even in a separate group of its own. The choice of where to place hydrogen often depends on the properties being emphasized and the purpose of the periodic table.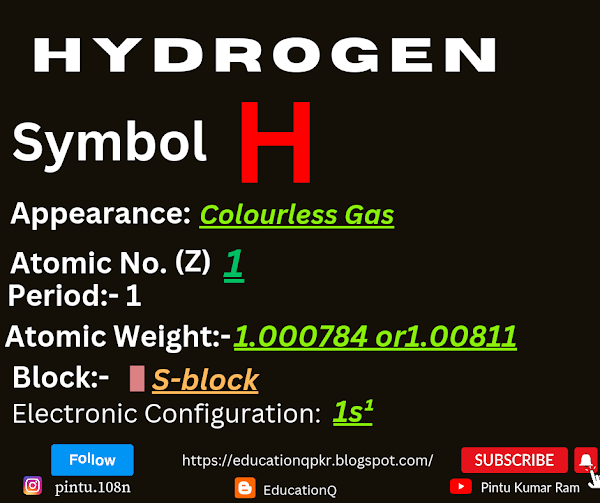


Very nice sir it help me a lot ☺️
ReplyDeleteThanks, stay focuss you will get lot of knowledge here.
Delete